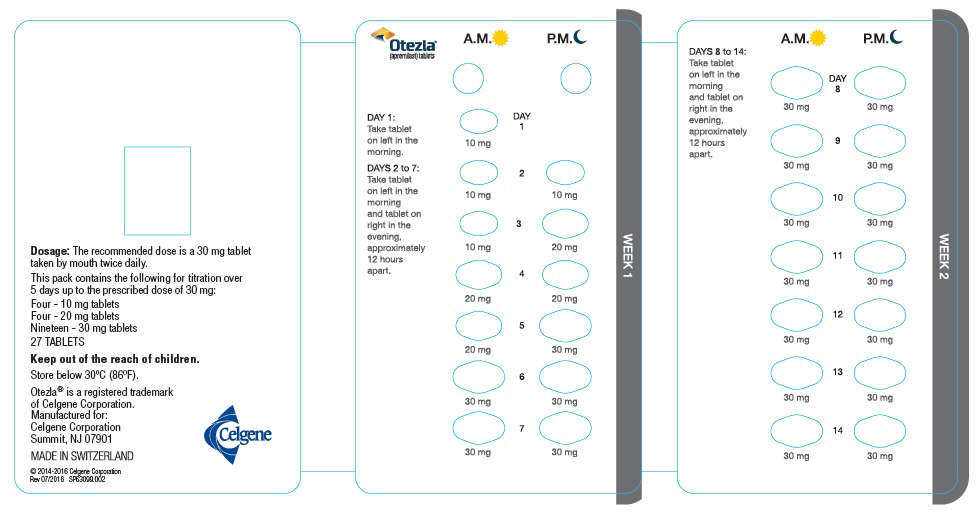
otezla starter pack directions
Dispense a Starter Pack in-office 14-day sample or through your specialty pharmacy 28-day pack Otezla has no initial or routine label-required lab monitoring 1. Otezla is contraindicated in patients with a known hypersensitivity to apremilast or to any of the.

These Highlights Do Not Include All The Information Needed To Use Otezla Safely And Effectively See Full Prescribing Information For Otezla Otezla Apremilast Tablets For Oral Use Initial U S Approval 2014


Get Started With Otezla Dosing Information Otezla Apremilast Healthcare Professional Site

Find Out How To Start Patients On Otezla Otezla Apremilast Healthcare Professional Site
These Highlights Do Not Include All The Information Needed To Use Otezla Safely And Effectively See Full Prescribing Information For Otezla Otezla Apremilast Tablets For Oral Use Initial U S Approval 2014

Apremilast Uses Side Effects Dosage Interactions

Get Started With Otezla Dosing Information Otezla Apremilast Healthcare Professional Site

These Highlights Do Not Include All The Information Needed To Use Otezla Safely And Effectively See Full Prescribing Information For Otezla Otezla Apremilast Tablets For Oral Use Initial U S Approval 2014

Otezla Starter Oral Uses Side Effects Interactions Pictures Warnings Dosing Webmd

These Highlights Do Not Include All The Information Needed To Use Otezla Safely And Effectively See Full Prescribing Information For Otezla Otezla Apremilast Tablets For Oral Use Initial U S Approval 2014

Otezla Starter Oral Uses Side Effects Interactions Pictures Warnings Dosing Webmd